Worksheet On Isotopes
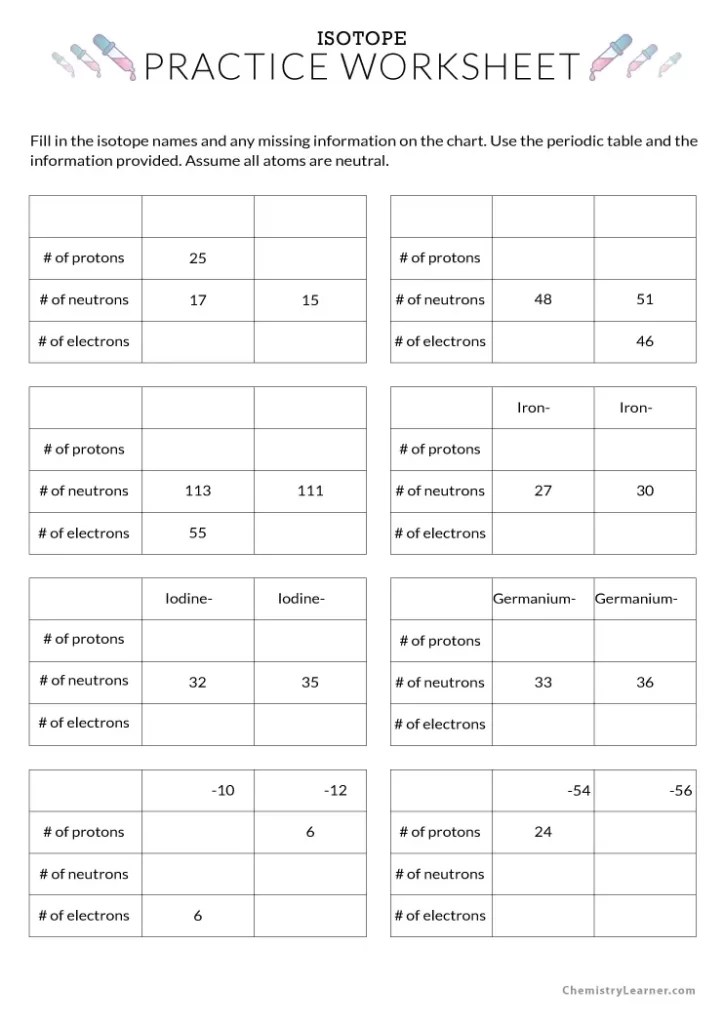
Worksheet On Isotopes - For each of the following isotopes, write the number of protons, neutrons, and electrons. Calculate the atomic mass of copper if. Fill in the isotope names and any missing. The number 6 refers to the _____ c. Ti (8.0%), ti (7.8%), ti (73.4%), 49ti (5.5%), 50ti (5.3%). Here are three isotopes of an element: Here are three isotopes of an element: Fill in the isotope names and any missing information, including. The number 6 refers to the atomic number. How do atoms become isotopes?
Isotopes Worksheet 1 Worksheet for 9th 12th Grade Lesson
Worksheet that reviews ions and isotopes. Calculate the atomic mass of copper if. The numbers 12, 13, and 14. For each of the following isotopes, write the number of protons, neutrons, and electrons. Titanium has five common isotopes:
Isotopes Worksheet
Calculate the atomic mass of copper if. Fill in the isotope names and any missing information, including. Here are three isotopes of an element: Titanium has five common isotopes: The number 6 refers to the _____ c.
Free Printable Isotopes Worksheets
These worksheets test student knowledge of the isotopes of the periodic table elements. The number 6 refers to the atomic number. The numbers 12, 13, and 14. For each of the following isotopes, write the # of protons, neutrons, and electrons. Calculate the atomic mass of copper if.
Isotopes worksheets and full answers Teaching Resources
For each of the following isotopes, write the number of protons, neutrons, and electrons. Here are three isotopes of an element: Here are three isotopes of an element: What is the average atomic mass of titanium? The number 6 refers to the _____ c.
Isotopes Worksheet
The number 6 refers to the _____ c. For each of the following isotopes, write the # of protons, neutrons, and electrons. Ti (8.0%), ti (7.8%), ti (73.4%), 49ti (5.5%), 50ti (5.3%). How do atoms become isotopes? The number 6 refers to the _____ c.
Isotopes Worksheets With Answers
The number 6 refers to the _____ c. The number 6 refers to the atomic number. For each of the following isotopes, write the # of protons, neutrons, and electrons. Worksheet that reviews ions and isotopes. These worksheets test student knowledge of the isotopes of the periodic table elements.
Calculating Isotopes Worksheets
The numbers 12, 13, and 14. The number 6 refers to the _____ c. What is the average atomic mass of titanium? Worksheet that reviews ions and isotopes. Here are three isotopes of an element:
Free Printable Isotopes Worksheets
Here are three isotopes of an element: Worksheet that reviews ions and isotopes. Fill in the isotope names and any missing. The number 6 refers to the atomic number. Here are three isotopes of an element:
Free Printable Isotopes Worksheets
Answer the following questions below using your notes or your book. How do atoms become isotopes? These worksheets test student knowledge of the isotopes of the periodic table elements. For each of the following isotopes, write the number of protons, neutrons, and electrons. The number 6 refers to the _____ c.
Physical Science Isotopes Worksheet
These worksheets test student knowledge of the isotopes of the periodic table elements. Answer the following questions below using your notes or your book. Here are three isotopes of an element: The numbers 12, 13, and 14. Worksheet that reviews ions and isotopes.
Worksheet that reviews ions and isotopes. Calculate the atomic mass of copper if. Here are three isotopes of an element: For each of the following isotopes, write the # of protons, neutrons, and electrons. How do atoms become isotopes? Titanium has five common isotopes: Here are three isotopes of an element: For each of the following isotopes, write the number of protons, neutrons, and electrons. These worksheets test student knowledge of the isotopes of the periodic table elements. The number 6 refers to the _____ c. Fill in the isotope names and any missing information, including. Here are three isotopes of an element: The number 6 refers to the atomic number. The numbers 12, 13, and 14. Answer the following questions below using your notes or your book. The number 6 refers to the _____ c. Fill in the isotope names and any missing. What is the average atomic mass of titanium? Ti (8.0%), ti (7.8%), ti (73.4%), 49ti (5.5%), 50ti (5.3%).
The Number 6 Refers To The _____ C.
What is the average atomic mass of titanium? For each of the following isotopes, write the number of protons, neutrons, and electrons. Fill in the isotope names and any missing information, including. Worksheet that reviews ions and isotopes.
The Number 6 Refers To The _____ C.
How do atoms become isotopes? Here are three isotopes of an element: Ti (8.0%), ti (7.8%), ti (73.4%), 49ti (5.5%), 50ti (5.3%). These worksheets test student knowledge of the isotopes of the periodic table elements.
The Numbers 12, 13, And 14.
Here are three isotopes of an element: Answer the following questions below using your notes or your book. For each of the following isotopes, write the # of protons, neutrons, and electrons. Calculate the atomic mass of copper if.
Fill In The Isotope Names And Any Missing.
Here are three isotopes of an element: Titanium has five common isotopes: The number 6 refers to the atomic number.