Moles Of Moles Worksheet
Moles Of Moles Worksheet - Use a separate piece of paper, show all work, and circle your. Mole calculation worksheet 1) how many moles are in 15 grams of lithium? The mole is one of the most important concepts in chemistry, as it allows for quantitative calculation of amounts of substances that may take part in. A) calculate the mass of a single 2he atom (2 protons, 2 neutrons, 2 electrons). Mole worksheet #2 make the following conversions using unit analysis. Calculate the molar mass of the substance. There are three definitions (equalities) of mole. There are three definitions (equalities) of mole. Each definition can be written as a set of two conversion factors. _____ now that you know how.
Worksheet Mole/Mole Problems Worksheet
_____ now that you know how. Use a separate piece of paper, show all work, and circle your. Mole worksheet #2 make the following conversions using unit analysis. Calculate the molar mass of the substance. Mole calculation worksheet 1) how many moles are in 15 grams of lithium?
Mole Worksheet 1 moles ↔ particles
Mole worksheet #2 make the following conversions using unit analysis. Each definition can be written as a set of two conversion factors. Calculate the molar mass of the substance. Mole calculation worksheet 1) how many moles are in 15 grams of lithium? The mole as a unit of mass define the term molar mass (worksheet #1):
Mole Ratios Worksheet with Answers Chemistry Exercises Worksheets Library
Mole worksheet #2 make the following conversions using unit analysis. There are three definitions (equalities) of mole. The mole is one of the most important concepts in chemistry, as it allows for quantitative calculation of amounts of substances that may take part in. A) calculate the mass of a single 2he atom (2 protons, 2 neutrons, 2 electrons). Mole calculation.
Mole/Mole Problems Worksheet Worksheets Library
Calculate the molar mass of the substance. Mole calculation worksheet 1) how many moles are in 15 grams of lithium? _____ now that you know how. 1 mole = molar mass (could be atomic mass from periodic table or molecular mass) 1 mole = 22.4 l of a gas at stp ( you do not need to worry. The mole.
Worksheets Mole Mole Problems
The mole as a unit of mass define the term molar mass (worksheet #1): Calculate the molar mass of the substance. Mole worksheet #2 make the following conversions using unit analysis. 1 mole = molar mass (could be atomic mass from periodic table or molecular mass) 1 mole = 22.4 l of a gas at stp ( you do not.
Chemistry Mole Calculation Worksheet Interactive worksheet
There are three definitions (equalities) of mole. Mole worksheet #2 make the following conversions using unit analysis. Calculate the molar mass of the substance. The mole as a unit of mass define the term molar mass (worksheet #1): _____ now that you know how.
Moles And Molar Mass Worksheet
A) calculate the mass of a single 2he atom (2 protons, 2 neutrons, 2 electrons). Mole worksheet #2 make the following conversions using unit analysis. Mole calculation worksheet 1) how many moles are in 15 grams of lithium? 1 mole = molar mass (could be atomic mass from periodic table or molecular mass) 1 mole = 22.4 l of a.
Moles Worksheet
Calculate the molar mass of the substance. _____ now that you know how. There are three definitions (equalities) of mole. Each definition can be written as a set of two conversion factors. Each definition can be written as a set of two conversion factors.
Free Printable Moles Worksheets
1 mole = molar mass (could be atomic mass from periodic table or molecular mass) 1 mole = 22.4 l of a gas at stp ( you do not need to worry. 2) how many grams are in 2.4 moles of sulfur? There are three definitions (equalities) of mole. The mole is one of the most important concepts in chemistry,.
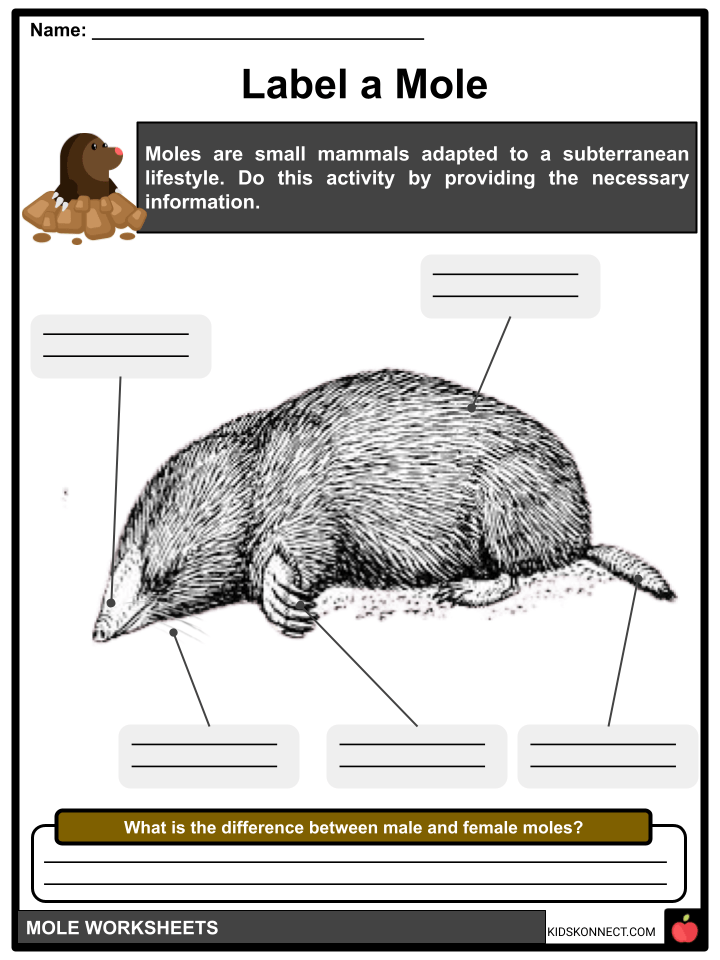
Mole Facts & Worksheets KidsKonnect
2) how many grams are in 2.4 moles of sulfur? Use a separate piece of paper, show all work, and circle your. The mole is one of the most important concepts in chemistry, as it allows for quantitative calculation of amounts of substances that may take part in. The mole as a unit of mass define the term molar mass.
Mole worksheet #2 make the following conversions using unit analysis. There are three definitions (equalities) of mole. Each definition can be written as a set of two conversion factors. Mole calculation worksheet 1) how many moles are in 15 grams of lithium? Calculate the molar mass of the substance. There are three definitions (equalities) of mole. 2) how many grams are in 2.4 moles of sulfur? The mole as a unit of mass define the term molar mass (worksheet #1): A) calculate the mass of a single 2he atom (2 protons, 2 neutrons, 2 electrons). The mole is one of the most important concepts in chemistry, as it allows for quantitative calculation of amounts of substances that may take part in. _____ now that you know how. Each definition can be written as a set of two conversion factors. Use a separate piece of paper, show all work, and circle your. 1 mole = molar mass (could be atomic mass from periodic table or molecular mass) 1 mole = 22.4 l of a gas at stp ( you do not need to worry.
There Are Three Definitions (Equalities) Of Mole.
A) calculate the mass of a single 2he atom (2 protons, 2 neutrons, 2 electrons). Mole worksheet #2 make the following conversions using unit analysis. Mole calculation worksheet 1) how many moles are in 15 grams of lithium? Use a separate piece of paper, show all work, and circle your.
The Mole As A Unit Of Mass Define The Term Molar Mass (Worksheet #1):
The mole is one of the most important concepts in chemistry, as it allows for quantitative calculation of amounts of substances that may take part in. 2) how many grams are in 2.4 moles of sulfur? Each definition can be written as a set of two conversion factors. 1 mole = molar mass (could be atomic mass from periodic table or molecular mass) 1 mole = 22.4 l of a gas at stp ( you do not need to worry.
There Are Three Definitions (Equalities) Of Mole.
Each definition can be written as a set of two conversion factors. Calculate the molar mass of the substance. _____ now that you know how.