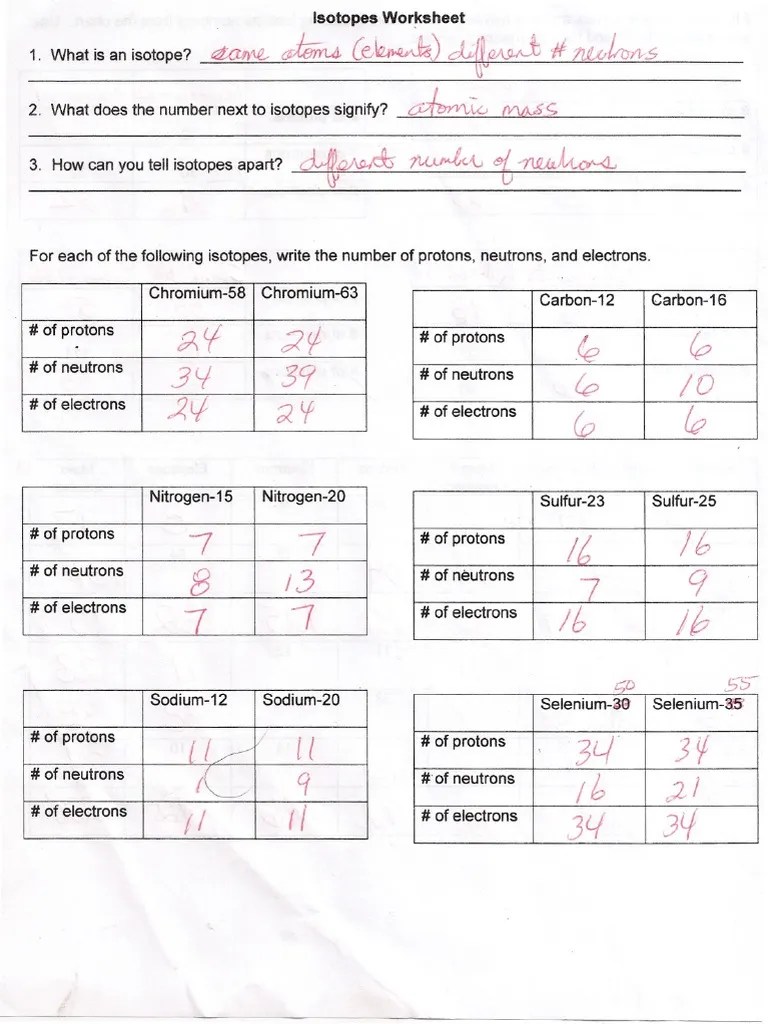
Isotopes Practice Worksheet
Isotopes Practice Worksheet - Calculate the atomic mass of copper if. Here are three isotopes of an element: Here are three isotopes of an element: For each of the following isotopes, write the # of protons, neutrons, and electrons. The number 6 refers to. What is the average atomic mass of titanium? 6 12c 6 13c 6 14c a. The number 6 refers to the _____ c. Argon has three naturally occurring isotopes: Based on argon’s reported atomic mass, which isotope.
Free Printable Ions and Isotopes Worksheets
Here are three isotopes of an element: What is the average atomic mass of titanium? 12c 14 13c c a. Fill in the isotope names and any missing information, including. The number 6 refers to the atomic number c.
Atomic Structure Ions And Isotopes Worksheet Worksheet Ions
These worksheets will help students grasp the essential principles behind isotopes and learn how to distinguish between different isotopes. The number 6 refers to the atomic number c. The number 6 refers to the _____ c. Here are three isotopes of an element: Fill in the isotope names and any missing information, including.
Isotopes Worksheet Chemistry Isotopes Worksheet
Here are three isotopes of an element: The number 6 refers to the atomic number c. Here are three isotopes of an element: Titanium has five common isotopes: Based on argon’s reported atomic mass, which isotope.
Ions And Isotopes Worksheet Free Worksheets Samples
Titanium has five common isotopes: What is the average atomic mass of titanium? Here are three isotopes of an element: The number 6 refers to the _____ c. Argon has three naturally occurring isotopes:
Free Printable Isotopes Worksheets
Fill in the isotope names and any missing information, including. The number 6 refers to the _____ c. Here are three isotopes of an element: These worksheets will help students grasp the essential principles behind isotopes and learn how to distinguish between different isotopes. Titanium has five common isotopes:
Isotope Practice Worksheet
Based on argon’s reported atomic mass, which isotope. The number 6 refers to. For each of the following isotopes, write the # of protons, neutrons, and electrons. Fill in the isotope names and any missing information, including. What is the average atomic mass of titanium?
Practice Isotope Calculations 2 Worksheet Answers
6 12c 6 13c 6 14c a. Titanium has five common isotopes: These worksheets will help students grasp the essential principles behind isotopes and learn how to distinguish between different isotopes. Here are three isotopes of an element: Here are three isotopes of an element:
Chemistry Isotopes Worksheet Printable Word Searches
Ti (8.0%), ti (7.8%), ti (73.4%), 49ti (5.5%), 50ti (5.3%). Fill in the isotope names and any missing information, including. These worksheets will help students grasp the essential principles behind isotopes and learn how to distinguish between different isotopes. Here are three isotopes of an element: Calculate the atomic mass of copper if.
Isotopes Worksheet
Argon has three naturally occurring isotopes: Here are three isotopes of an element: Calculate the atomic mass of copper if. Here are three isotopes of an element: Ti (8.0%), ti (7.8%), ti (73.4%), 49ti (5.5%), 50ti (5.3%).
Isotopes And Nuclear Chemistry Worksheet
Here are three isotopes of an element: Here are three isotopes of an element: 12c 14 13c c a. The number 6 refers to the _____ c. Based on argon’s reported atomic mass, which isotope.
12c 14 13c c a. Argon has three naturally occurring isotopes: For each of the following isotopes, write the # of protons, neutrons, and electrons. Fill in the isotope names and any missing information, including. The number 6 refers to the atomic number c. Here are three isotopes of an element: 6 12c 6 13c 6 14c a. Here are three isotopes of an element: Here are three isotopes of an element: The number 6 refers to. Calculate the atomic mass of copper if. Based on argon’s reported atomic mass, which isotope. The number 6 refers to the _____ c. Titanium has five common isotopes: What is the average atomic mass of titanium? These worksheets will help students grasp the essential principles behind isotopes and learn how to distinguish between different isotopes. Ti (8.0%), ti (7.8%), ti (73.4%), 49ti (5.5%), 50ti (5.3%).
Titanium Has Five Common Isotopes:
The number 6 refers to the atomic number c. Calculate the atomic mass of copper if. Fill in the isotope names and any missing information, including. The number 6 refers to the _____ c.
Here Are Three Isotopes Of An Element:
Ti (8.0%), ti (7.8%), ti (73.4%), 49ti (5.5%), 50ti (5.3%). What is the average atomic mass of titanium? 6 12c 6 13c 6 14c a. Here are three isotopes of an element:
12C 14 13C C A.
For each of the following isotopes, write the # of protons, neutrons, and electrons. Based on argon’s reported atomic mass, which isotope. The number 6 refers to. Argon has three naturally occurring isotopes:
These Worksheets Will Help Students Grasp The Essential Principles Behind Isotopes And Learn How To Distinguish Between Different Isotopes.
Here are three isotopes of an element: