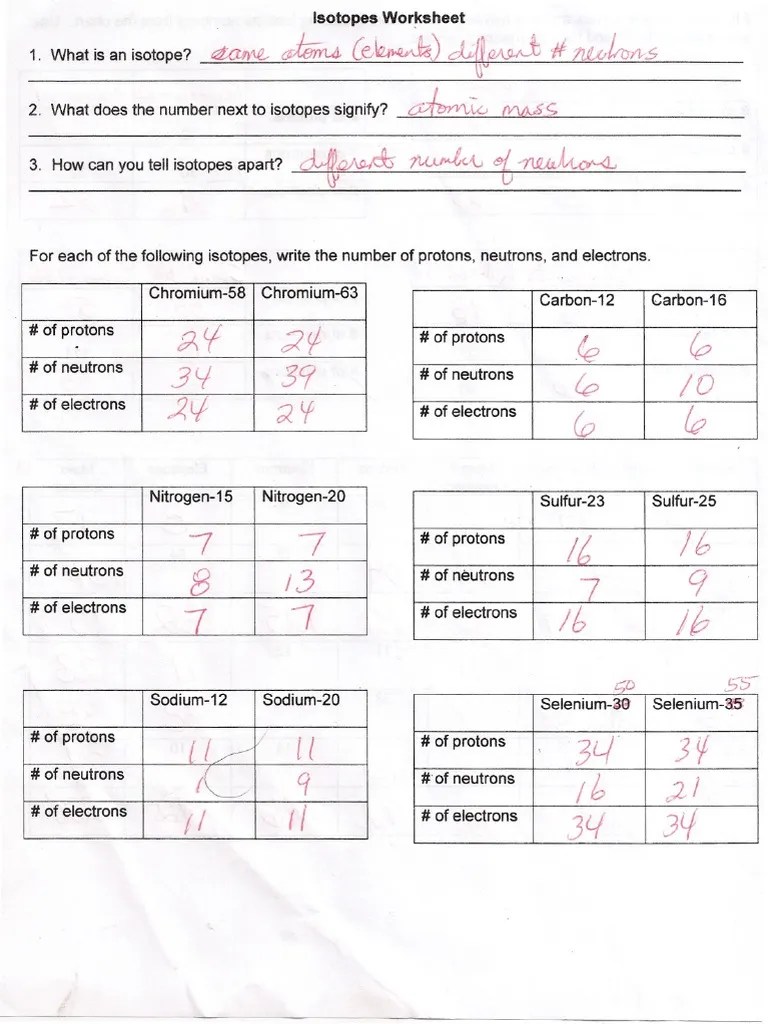
Isotope Practice Worksheet Answers
Isotope Practice Worksheet Answers - Argon has three naturally occurring isotopes: What is the average atomic mass of titanium? Here are three isotopes of an element: Titanium has five common isotopes: Calculate the atomic mass of copper if. Use the following examples to help answer the questions below: An isotope of chromium that has 3 more neutrons than 54. The number 6 refers to the _____ c. Ti (8.0%), ti (7.8%), ti (73.4%), 49ti (5.5%), 50ti (5.3%). Use the equation for atomic mass to complete the following problems.
Practice Isotope Calculations 2 Worksheet Answers
Titanium has five common isotopes: Here are three isotopes of an element: Argon has three naturally occurring isotopes: Given the number of protons, neutrons, and electrons, what would be the mass number and the charge number of each isotope? Use the equation for atomic mass to complete the following problems.
Isotopes Worksheet
Ti (8.0%), ti (7.8%), ti (73.4%), 49ti (5.5%), 50ti (5.3%). On this website, we have prepared dozens of free isotope worksheets for extra practice. What is the average atomic mass of titanium? An isotope of chromium that has 3 more neutrons than 54. These chemistry worksheets will give students a.
Practice Isotope Calculations 2 Worksheet Answers
Ti (8.0%), ti (7.8%), ti (73.4%), 49ti (5.5%), 50ti (5.3%). These chemistry worksheets will give students a. Here are three isotopes of an element: Show your work, and be. The number 6 refers to the _____ c.
Practice Isotope Calculations 2 Answer Key Practice Isotope
Show your work, and be. Given the number of protons, neutrons, and electrons, what would be the mass number and the charge number of each isotope? Calculate the atomic mass of copper if. Ti (8.0%), ti (7.8%), ti (73.4%), 49ti (5.5%), 50ti (5.3%). Use the equation for atomic mass to complete the following problems.
50 Isotope Practice Worksheet Answer Key
Argon has three naturally occurring isotopes: Show your work, and be. An isotope of chromium that has 3 more neutrons than 54. Ti (8.0%), ti (7.8%), ti (73.4%), 49ti (5.5%), 50ti (5.3%). Calculate the atomic mass of copper if.
Practice Isotope Calculations 2 Answer Key Practice Isotope
Show your work, and be. Argon has three naturally occurring isotopes: Ti (8.0%), ti (7.8%), ti (73.4%), 49ti (5.5%), 50ti (5.3%). Given the number of protons, neutrons, and electrons, what would be the mass number and the charge number of each isotope? Titanium has five common isotopes:
Practice Isotope Calculations 2 Answer Key Practice Isotope
Argon has three naturally occurring isotopes: On this website, we have prepared dozens of free isotope worksheets for extra practice. Write complete symbols (a symbol) for the following: Here are three isotopes of an element: Use the following examples to help answer the questions below:
Chemistry Worksheet Isotope Notation Chemistry Worksheet Iso
Calculate the atomic mass of copper if. Use the following examples to help answer the questions below: Given the number of protons, neutrons, and electrons, what would be the mass number and the charge number of each isotope? An isotope of chromium that has 3 more neutrons than 54. On this website, we have prepared dozens of free isotope worksheets.
Isotopes Practice Worksheet Isotopes Worksheet Answers Key L
Use the following examples to help answer the questions below: Use the equation for atomic mass to complete the following problems. Here are three isotopes of an element: The number 6 refers to the _____ c. Given the number of protons, neutrons, and electrons, what would be the mass number and the charge number of each isotope?
Isotope Practice Worksheet Answers
These chemistry worksheets will give students a. The number 6 refers to the _____ c. An isotope of chromium that has 3 more neutrons than 54. Argon has three naturally occurring isotopes: What is the average atomic mass of titanium?
Write complete symbols (a symbol) for the following: Argon has three naturally occurring isotopes: Show your work, and be. Titanium has five common isotopes: Calculate the atomic mass of copper if. Use the following examples to help answer the questions below: These chemistry worksheets will give students a. On this website, we have prepared dozens of free isotope worksheets for extra practice. Given the number of protons, neutrons, and electrons, what would be the mass number and the charge number of each isotope? What is the average atomic mass of titanium? Ti (8.0%), ti (7.8%), ti (73.4%), 49ti (5.5%), 50ti (5.3%). Use the equation for atomic mass to complete the following problems. The number 6 refers to the _____ c. Here are three isotopes of an element: An isotope of chromium that has 3 more neutrons than 54.
What Is The Average Atomic Mass Of Titanium?
Use the equation for atomic mass to complete the following problems. Calculate the atomic mass of copper if. Here are three isotopes of an element: Show your work, and be.
Titanium Has Five Common Isotopes:
The number 6 refers to the _____ c. An isotope of chromium that has 3 more neutrons than 54. Write complete symbols (a symbol) for the following: These chemistry worksheets will give students a.
Ti (8.0%), Ti (7.8%), Ti (73.4%), 49Ti (5.5%), 50Ti (5.3%).
Argon has three naturally occurring isotopes: Given the number of protons, neutrons, and electrons, what would be the mass number and the charge number of each isotope? On this website, we have prepared dozens of free isotope worksheets for extra practice. Use the following examples to help answer the questions below: