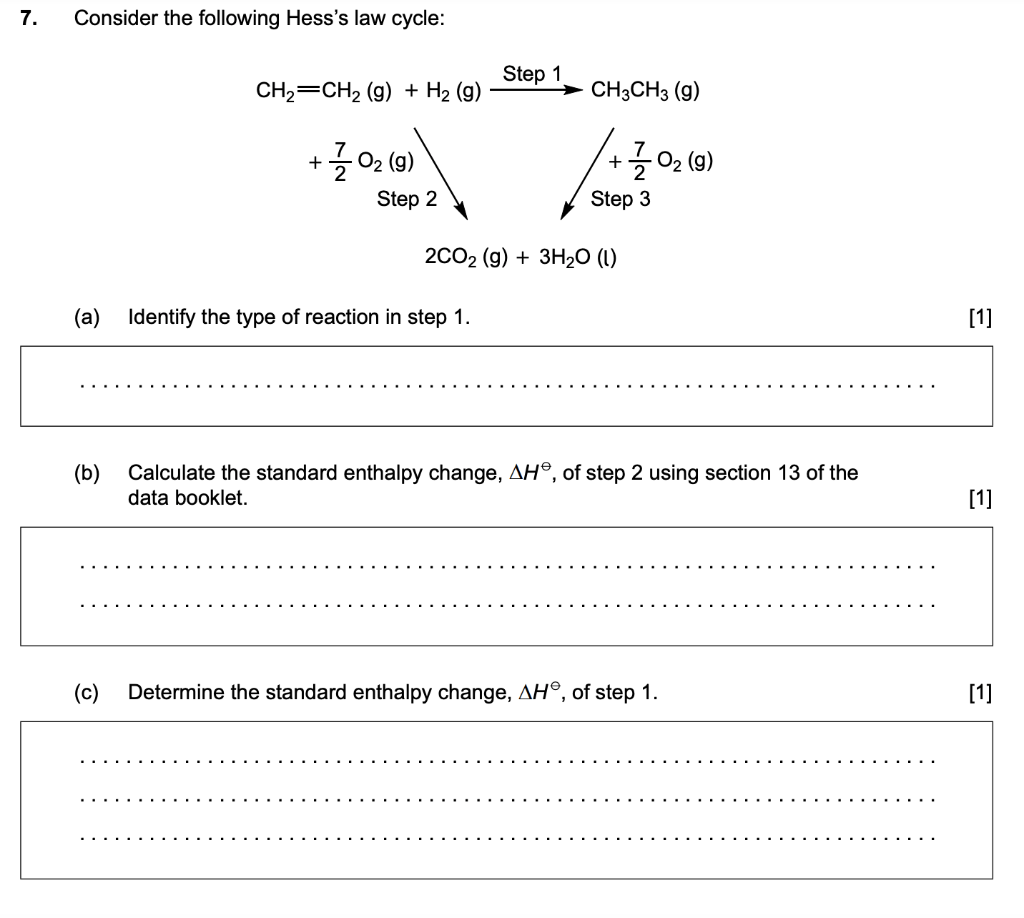
Hess Law Worksheet
Hess Law Worksheet - Our hess's law worksheets contain a set of practice problems involving hess's law, in which students reverse, multiply, or divide reactions to get them to combine into a single equation. C 2h 4 (g) + h 2 (g) à c 2h 6 (g), from the following data: Calculate the standard enthalpy change, δho, for the formation of 1 mol of strontium carbonate (the material that gives the red color in fireworks) from its elements. Refer to the table of heats of formation for the individual step. C 2h 4 (g) + 3 o 2. Chem 12 hess’s law worksheet 1. Calculate the δh for the reaction: Complete these questions using the additivity of heats method. Hess’s law can be used to determine the enthalpy of a reaction by manipulating known thermochemical equations that could be used as a reaction pathway to the desired reaction.
Hess law worksheet CHEM120 Studocu
C 2h 4 (g) + h 2 (g) à c 2h 6 (g), from the following data: Our hess's law worksheets contain a set of practice problems involving hess's law, in which students reverse, multiply, or divide reactions to get them to combine into a single equation. Hess’s law can be used to determine the enthalpy of a reaction by.
Chemsheets Hess Law 2 Answers
Calculate the standard enthalpy change, δho, for the formation of 1 mol of strontium carbonate (the material that gives the red color in fireworks) from its elements. Refer to the table of heats of formation for the individual step. Complete these questions using the additivity of heats method. C 2h 4 (g) + h 2 (g) à c 2h 6.
Free Printable Hess's Law Worksheets
C 2h 4 (g) + 3 o 2. Chem 12 hess’s law worksheet 1. Our hess's law worksheets contain a set of practice problems involving hess's law, in which students reverse, multiply, or divide reactions to get them to combine into a single equation. Calculate the standard enthalpy change, δho, for the formation of 1 mol of strontium carbonate (the.
Hess Law Practice PDF Chemical Substances Branches Of Worksheets Library
Calculate the standard enthalpy change, δho, for the formation of 1 mol of strontium carbonate (the material that gives the red color in fireworks) from its elements. C 2h 4 (g) + 3 o 2. C 2h 4 (g) + h 2 (g) à c 2h 6 (g), from the following data: Chem 12 hess’s law worksheet 1. Complete these.
Hess's Law Worksheet 2
Calculate the standard enthalpy change, δho, for the formation of 1 mol of strontium carbonate (the material that gives the red color in fireworks) from its elements. Refer to the table of heats of formation for the individual step. Hess’s law can be used to determine the enthalpy of a reaction by manipulating known thermochemical equations that could be used.
Hess Law A Level Chemistry Questions
Refer to the table of heats of formation for the individual step. C 2h 4 (g) + 3 o 2. Complete these questions using the additivity of heats method. Our hess's law worksheets contain a set of practice problems involving hess's law, in which students reverse, multiply, or divide reactions to get them to combine into a single equation. Calculate.
30++ Hess's Law Worksheet Answers Worksheets Decoomo
Chem 12 hess’s law worksheet 1. Refer to the table of heats of formation for the individual step. Our hess's law worksheets contain a set of practice problems involving hess's law, in which students reverse, multiply, or divide reactions to get them to combine into a single equation. Calculate the standard enthalpy change, δho, for the formation of 1 mol.
Hess's+law Practice Problems Hesss Law Practice Problems Given the following equations and Ho
Calculate the δh for the reaction: Hess’s law can be used to determine the enthalpy of a reaction by manipulating known thermochemical equations that could be used as a reaction pathway to the desired reaction. C 2h 4 (g) + 3 o 2. Our hess's law worksheets contain a set of practice problems involving hess's law, in which students reverse,.
Hess' Law Worksheet Hess' Law chemistry questions and answers Studocu
Refer to the table of heats of formation for the individual step. Calculate the standard enthalpy change, δho, for the formation of 1 mol of strontium carbonate (the material that gives the red color in fireworks) from its elements. C 2h 4 (g) + h 2 (g) à c 2h 6 (g), from the following data: C 2h 4 (g).
Ap Chemistry Hess's Law Worksheet
Calculate the standard enthalpy change, δho, for the formation of 1 mol of strontium carbonate (the material that gives the red color in fireworks) from its elements. C 2h 4 (g) + h 2 (g) à c 2h 6 (g), from the following data: Refer to the table of heats of formation for the individual step. Hess’s law can be.
C 2h 4 (g) + h 2 (g) à c 2h 6 (g), from the following data: Refer to the table of heats of formation for the individual step. Our hess's law worksheets contain a set of practice problems involving hess's law, in which students reverse, multiply, or divide reactions to get them to combine into a single equation. Hess’s law can be used to determine the enthalpy of a reaction by manipulating known thermochemical equations that could be used as a reaction pathway to the desired reaction. Complete these questions using the additivity of heats method. Calculate the standard enthalpy change, δho, for the formation of 1 mol of strontium carbonate (the material that gives the red color in fireworks) from its elements. Chem 12 hess’s law worksheet 1. C 2h 4 (g) + 3 o 2. Calculate the δh for the reaction:
Chem 12 Hess’s Law Worksheet 1.
C 2h 4 (g) + h 2 (g) à c 2h 6 (g), from the following data: Complete these questions using the additivity of heats method. C 2h 4 (g) + 3 o 2. Hess’s law can be used to determine the enthalpy of a reaction by manipulating known thermochemical equations that could be used as a reaction pathway to the desired reaction.
Calculate The Standard Enthalpy Change, Δho, For The Formation Of 1 Mol Of Strontium Carbonate (The Material That Gives The Red Color In Fireworks) From Its Elements.
Calculate the δh for the reaction: Our hess's law worksheets contain a set of practice problems involving hess's law, in which students reverse, multiply, or divide reactions to get them to combine into a single equation. Refer to the table of heats of formation for the individual step.