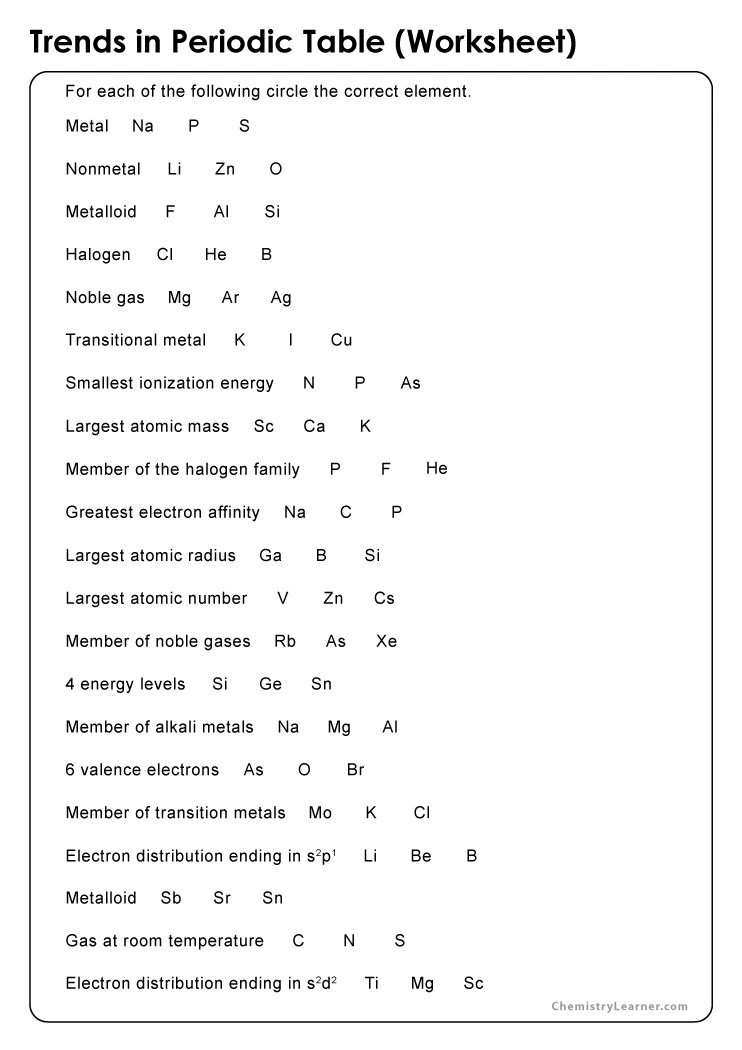
Chemistry Periodic Trends Worksheet Answers
Chemistry Periodic Trends Worksheet Answers - Discuss the importance of mendeleev’s. If you are struggling with problems concerning periodic trends, please don’t be worried, we. Knowing the trends in atomic and ionic sizes, ionization energies, and electron affinities aids in understanding chemical behavior and the nature of chemical bonds. Explain why you made these choices: What trend would you predict for the binding energies of valence electrons moving down a. All of the elements are in the same period. Answers for comparing tendencies to gain electrons here are answers to the exercises. Cu k ni br a. Explain why this trend occurs. What trends do you notice for the atomic radii of period 3?
The Essential Guide to Understanding Periodic Trends Worksheet Answers
Knowing the trends in atomic and ionic sizes, ionization energies, and electron affinities aids in understanding chemical behavior and the nature of chemical bonds. Discuss the importance of mendeleev’s. The trend in atomic radius as you go across a period is. If you are struggling with problems concerning periodic trends, please don’t be worried, we. Cu k ni br a.
Free Printable Periodic Trends Worksheets
Explain why you made these choices: If you are struggling with problems concerning periodic trends, please don’t be worried, we. The trend in atomic radius as you go across a period is. Circle the element with the largest atomic radius and put a square around the element with the smallest atomic radius: Cu k ni br a.
PERIODIC TRENDS WORKSHEET ANSWERS
Explain why this trend occurs. Discuss the importance of mendeleev’s. If you are struggling with problems concerning periodic trends, please don’t be worried, we. Circle the element with the largest atomic radius and put a square around the element with the smallest atomic radius: Knowing the trends in atomic and ionic sizes, ionization energies, and electron affinities aids in understanding.
Periodic Trends Practice Worksheet Answers Proworksheet.my.id
Discuss the importance of mendeleev’s. The trend in atomic radius as you go across a period is. Cu k ni br a. Answers for comparing tendencies to gain electrons here are answers to the exercises. If you are struggling with problems concerning periodic trends, please don’t be worried, we.
Graphing Periodic Trends Activity Answer Key Worksheet Periodic
Cu k ni br a. Knowing the trends in atomic and ionic sizes, ionization energies, and electron affinities aids in understanding chemical behavior and the nature of chemical bonds. The trend in atomic radius as you go across a period is. Circle the element with the largest atomic radius and put a square around the element with the smallest atomic.
Periodic Trends Worksheet Answers
What trends do you notice for the atomic radii of period 3? Discuss the importance of mendeleev’s. What trend would you predict for the binding energies of valence electrons moving down a. Cu k ni br a. Explain why this trend occurs.
Periodic Trends Worksheet Answer Key 2 Name Studocu Worksheets
What trends do you notice for the atomic radii of period 3? Explain why you made these choices: Circle the element with the largest atomic radius and put a square around the element with the smallest atomic radius: If you are struggling with problems concerning periodic trends, please don’t be worried, we. Knowing the trends in atomic and ionic sizes,.
Chemistry Periodic Table Trends Worksheet Answer Key Periodic Table
Answers for comparing tendencies to gain electrons here are answers to the exercises. What trends do you notice for the atomic radii of period 3? What trend would you predict for the binding energies of valence electrons moving down a. Explain why this trend occurs. Explain why you made these choices:
15 Periodic Trends Answer Key PDF Worksheets Library
Knowing the trends in atomic and ionic sizes, ionization energies, and electron affinities aids in understanding chemical behavior and the nature of chemical bonds. What trends do you notice for the atomic radii of period 3? All of the elements are in the same period. Cu k ni br a. The trend in atomic radius as you go across a.
Periodic Table Trends Worksheet With Answers Matttroy
Discuss the importance of mendeleev’s. What trend would you predict for the binding energies of valence electrons moving down a. Answers for comparing tendencies to gain electrons here are answers to the exercises. Explain why this trend occurs. Explain why you made these choices:
If you are struggling with problems concerning periodic trends, please don’t be worried, we. Knowing the trends in atomic and ionic sizes, ionization energies, and electron affinities aids in understanding chemical behavior and the nature of chemical bonds. Explain why you made these choices: Explain why this trend occurs. What trends do you notice for the atomic radii of period 3? Circle the element with the largest atomic radius and put a square around the element with the smallest atomic radius: What trend would you predict for the binding energies of valence electrons moving down a. Discuss the importance of mendeleev’s. The trend in atomic radius as you go across a period is. Cu k ni br a. All of the elements are in the same period. Answers for comparing tendencies to gain electrons here are answers to the exercises.
If You Are Struggling With Problems Concerning Periodic Trends, Please Don’t Be Worried, We.
All of the elements are in the same period. Cu k ni br a. The trend in atomic radius as you go across a period is. Explain why this trend occurs.
Circle The Element With The Largest Atomic Radius And Put A Square Around The Element With The Smallest Atomic Radius:
Discuss the importance of mendeleev’s. Explain why you made these choices: What trends do you notice for the atomic radii of period 3? Knowing the trends in atomic and ionic sizes, ionization energies, and electron affinities aids in understanding chemical behavior and the nature of chemical bonds.
What Trend Would You Predict For The Binding Energies Of Valence Electrons Moving Down A.
Answers for comparing tendencies to gain electrons here are answers to the exercises.