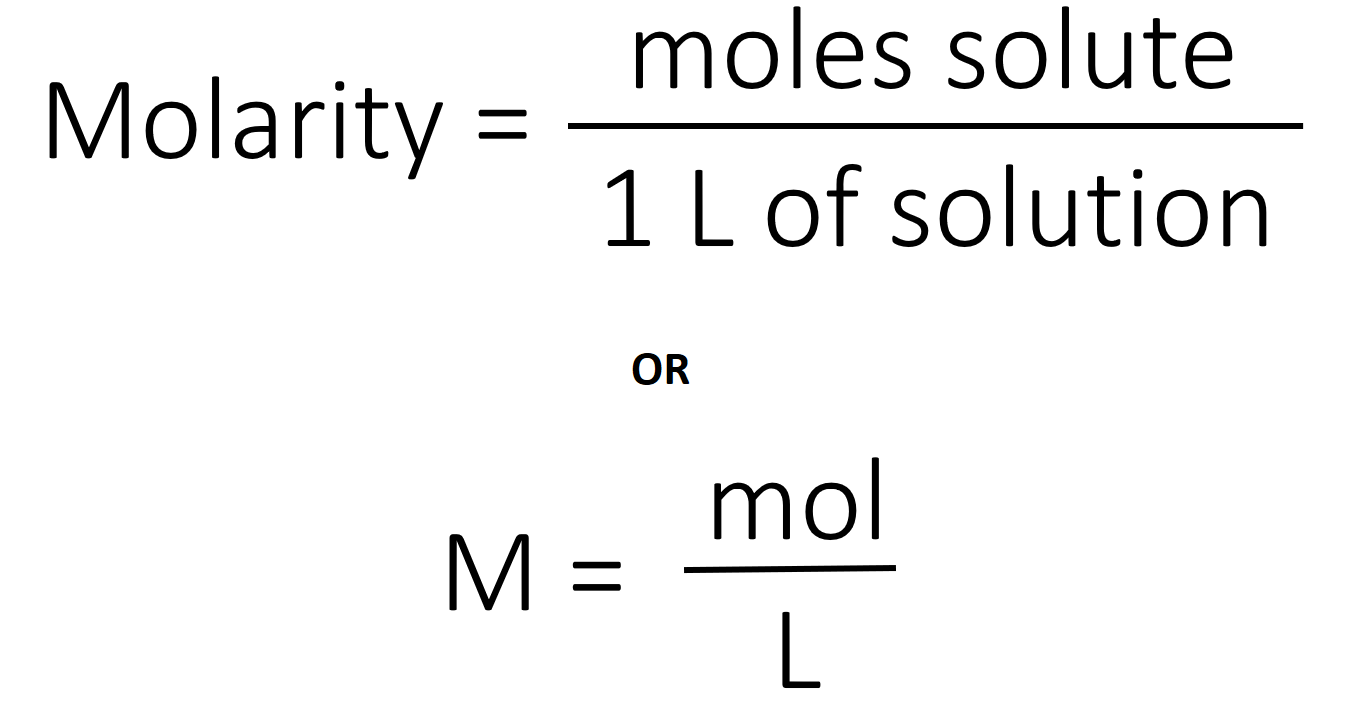Chemistry Molarity Worksheet
Chemistry Molarity Worksheet - In the first solution, 1.0 moles of sodium chloride is dissolved to make 1.0 liters of solution. What is the molarity of the solution? What is the molarity of 2.0 l of solution made from. What is the molarity of the following solutions given that: The molarity of a solution is defined as the moles of solute per liter of solution. • a _______________ quantitative description of solution concentration. From basic calculations to complex scenarios, these worksheets are designed to guide learners through various exercises that enhance their ability to calculate molarity, dilutions, and related concepts. Molarity extra practice worksheet 1. 1.0 moles of potassium fluoride is. How many moles of solute are present in 1.5 l of.
Molarity
• a _______________ quantitative description of solution concentration. What is the molarity of the solution? Molarity extra practice worksheet 1. What is the molarity of the following solutions given that: From basic calculations to complex scenarios, these worksheets are designed to guide learners through various exercises that enhance their ability to calculate molarity, dilutions, and related concepts.
Chemistry Molarity Of Solutions Worksheets
What is the molarity of 2.0 l of solution made from. From basic calculations to complex scenarios, these worksheets are designed to guide learners through various exercises that enhance their ability to calculate molarity, dilutions, and related concepts. What is the molarity of the following solutions given that: The molarity of a solution is defined as the moles of solute.
Calculating Molarity, Moles, and Volume A Chemistry Worksheet
The molarity of a solution is defined as the moles of solute per liter of solution. From basic calculations to complex scenarios, these worksheets are designed to guide learners through various exercises that enhance their ability to calculate molarity, dilutions, and related concepts. In the first solution, 1.0 moles of sodium chloride is dissolved to make 1.0 liters of solution..
Molarity Worksheet Answer Key
Molarity extra practice worksheet 1. From basic calculations to complex scenarios, these worksheets are designed to guide learners through various exercises that enhance their ability to calculate molarity, dilutions, and related concepts. In the first solution, 1.0 moles of sodium chloride is dissolved to make 1.0 liters of solution. What is the molarity of 2.0 l of solution made from..
Molarity_Worksheet 1 ans key Molar Concentration Magnesium
What is the molarity of the following solutions given that: What is the molarity of the solution? Molarity extra practice worksheet 1. 1.0 moles of potassium fluoride is. The molarity of a solution is defined as the moles of solute per liter of solution.
Molarity Worksheet Answers Chemistry Ivuyteq
Molarity extra practice worksheet 1. What is the molarity of the solution? What is the molarity of 2.0 l of solution made from. • a _______________ quantitative description of solution concentration. How many moles of solute are present in 1.5 l of.
Mole Ratio Worksheet
What is the molarity of the following solutions given that: From basic calculations to complex scenarios, these worksheets are designed to guide learners through various exercises that enhance their ability to calculate molarity, dilutions, and related concepts. • a _______________ quantitative description of solution concentration. How many moles of solute are present in 1.5 l of. What is the molarity.
Chemistry Molarity Of Solutions Worksheets
The molarity of a solution is defined as the moles of solute per liter of solution. Molarity extra practice worksheet 1. 1.0 moles of potassium fluoride is. What is the molarity of the solution? What is the molarity of the following solutions given that:
Calculating Molarity, Moles, and Volume A Chemistry Worksheet Made
• a _______________ quantitative description of solution concentration. What is the molarity of the following solutions given that: How many moles of solute are present in 1.5 l of. 1.0 moles of potassium fluoride is. What is the molarity of 2.0 l of solution made from.
Molarity Worksheet 1 Answer Key Chemistry
What is the molarity of the solution? The molarity of a solution is defined as the moles of solute per liter of solution. In the first solution, 1.0 moles of sodium chloride is dissolved to make 1.0 liters of solution. Molarity extra practice worksheet 1. What is the molarity of the following solutions given that:
What is the molarity of 2.0 l of solution made from. In the first solution, 1.0 moles of sodium chloride is dissolved to make 1.0 liters of solution. 1.0 moles of potassium fluoride is. How many moles of solute are present in 1.5 l of. What is the molarity of the following solutions given that: • a _______________ quantitative description of solution concentration. What is the molarity of the solution? Molarity extra practice worksheet 1. From basic calculations to complex scenarios, these worksheets are designed to guide learners through various exercises that enhance their ability to calculate molarity, dilutions, and related concepts. The molarity of a solution is defined as the moles of solute per liter of solution.
From Basic Calculations To Complex Scenarios, These Worksheets Are Designed To Guide Learners Through Various Exercises That Enhance Their Ability To Calculate Molarity, Dilutions, And Related Concepts.
Molarity extra practice worksheet 1. 1.0 moles of potassium fluoride is. The molarity of a solution is defined as the moles of solute per liter of solution. How many moles of solute are present in 1.5 l of.
What Is The Molarity Of The Following Solutions Given That:
What is the molarity of the solution? • a _______________ quantitative description of solution concentration. In the first solution, 1.0 moles of sodium chloride is dissolved to make 1.0 liters of solution. What is the molarity of 2.0 l of solution made from.