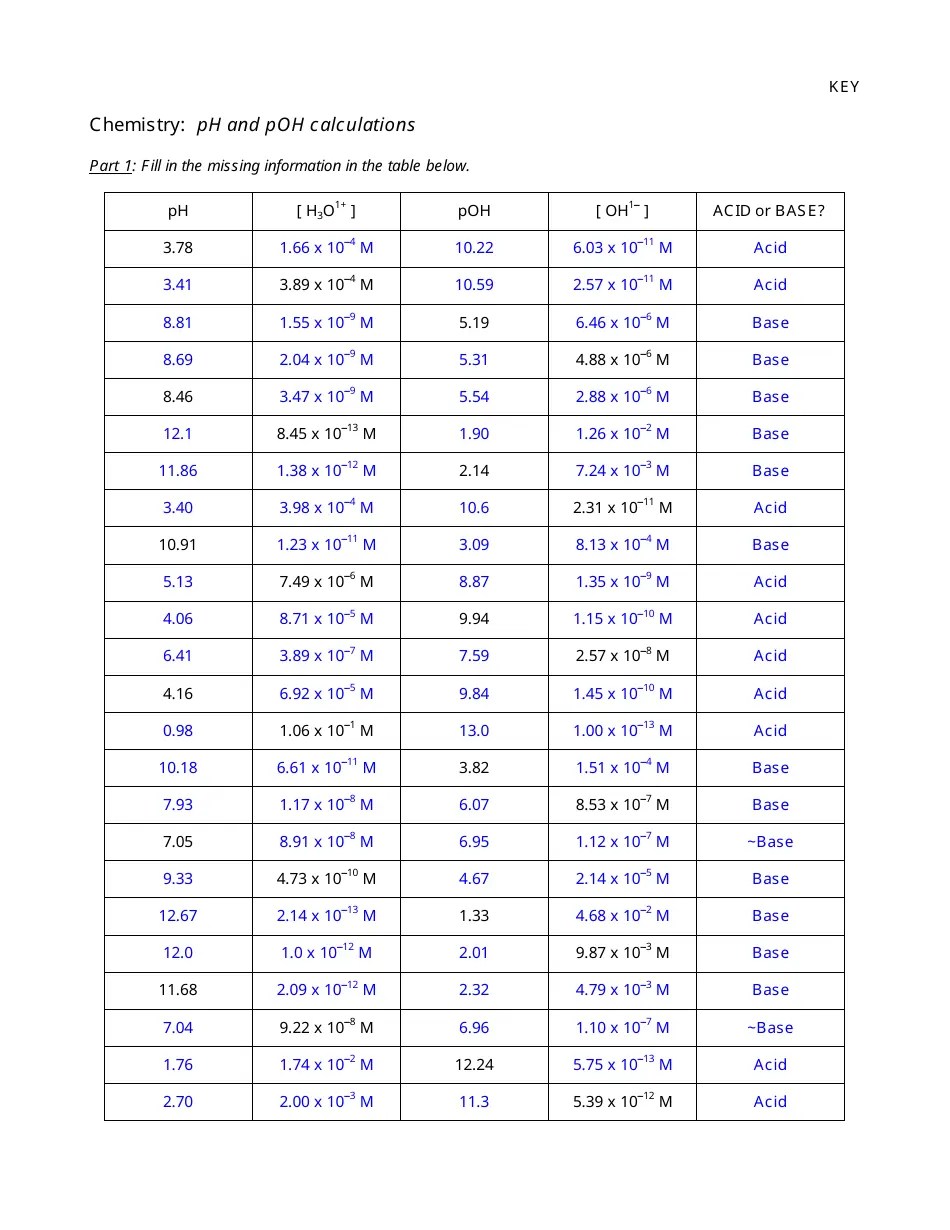
Calculating Ph And Poh Worksheet
Calculating Ph And Poh Worksheet - 2) what is the poh of a. What mass of naoh should be added to 300.0 ml of water in order to prepare a solution with a ph = 11.50. Write the equation for the dissociation of sulfuric acid. Calculating ph and poh worksheet w 335 everett community college tutoring center student support services program 1) what is the ph of a 0.0235 m hcl solution? What is the concentration of [h+] in a solution whose ph = 4.3? What is the poh of a solution that has a. Find the ph of a 0.00476 m hydrochloric acid solution. Write the equation for the dissociation of hydrochloric acid.
Ph And Poh Calculations Worksheet
What is the poh of a solution that has a. What is the concentration of [h+] in a solution whose ph = 4.3? What mass of naoh should be added to 300.0 ml of water in order to prepare a solution with a ph = 11.50. Write the equation for the dissociation of sulfuric acid. Calculating ph and poh worksheet.
SOLUTION Chemistry Ph and Poh Calculations Study Guide Studypool Worksheets Library
Find the ph of a 0.00476 m hydrochloric acid solution. What mass of naoh should be added to 300.0 ml of water in order to prepare a solution with a ph = 11.50. Calculating ph and poh worksheet w 335 everett community college tutoring center student support services program 1) what is the ph of a 0.0235 m hcl solution?.
Ph And Poh Calculations Worksheet 2
What mass of naoh should be added to 300.0 ml of water in order to prepare a solution with a ph = 11.50. Calculating ph and poh worksheet w 335 everett community college tutoring center student support services program 1) what is the ph of a 0.0235 m hcl solution? 2) what is the poh of a. What is the.
Ph And Poh Calculations Worksheet 2
Calculating ph and poh worksheet w 335 everett community college tutoring center student support services program 1) what is the ph of a 0.0235 m hcl solution? What mass of naoh should be added to 300.0 ml of water in order to prepare a solution with a ph = 11.50. What is the concentration of [h+] in a solution whose.
pH and pOH Practice Worksheet
What mass of naoh should be added to 300.0 ml of water in order to prepare a solution with a ph = 11.50. 2) what is the poh of a. Write the equation for the dissociation of hydrochloric acid. What is the concentration of [h+] in a solution whose ph = 4.3? Find the ph of a 0.00476 m hydrochloric.
Ph Calculations Worksheet With Work
Find the ph of a 0.00476 m hydrochloric acid solution. Calculating ph and poh worksheet w 335 everett community college tutoring center student support services program 1) what is the ph of a 0.0235 m hcl solution? 2) what is the poh of a. Write the equation for the dissociation of hydrochloric acid. What mass of naoh should be added.
Ph and Poh Calculations Chemistry Worksheet With Answers Download Printable PDF Templateroller
What is the poh of a solution that has a. What mass of naoh should be added to 300.0 ml of water in order to prepare a solution with a ph = 11.50. Find the ph of a 0.00476 m hydrochloric acid solution. 2) what is the poh of a. Calculating ph and poh worksheet w 335 everett community college.
Calculating Ph And Poh Worksheet
Find the ph of a 0.00476 m hydrochloric acid solution. What is the poh of a solution that has a. Write the equation for the dissociation of sulfuric acid. What mass of naoh should be added to 300.0 ml of water in order to prepare a solution with a ph = 11.50. Write the equation for the dissociation of hydrochloric.
Free Printable pH and pOH Worksheets
What is the poh of a solution that has a. What is the concentration of [h+] in a solution whose ph = 4.3? Write the equation for the dissociation of hydrochloric acid. 2) what is the poh of a. Calculating ph and poh worksheet w 335 everett community college tutoring center student support services program 1) what is the ph.
Ph and Poh Calculations Chemistry Worksheet With Answers Download Printable PDF Templateroller
Calculating ph and poh worksheet w 335 everett community college tutoring center student support services program 1) what is the ph of a 0.0235 m hcl solution? 2) what is the poh of a. Write the equation for the dissociation of hydrochloric acid. Write the equation for the dissociation of sulfuric acid. What mass of naoh should be added to.
2) what is the poh of a. Calculating ph and poh worksheet w 335 everett community college tutoring center student support services program 1) what is the ph of a 0.0235 m hcl solution? What mass of naoh should be added to 300.0 ml of water in order to prepare a solution with a ph = 11.50. Find the ph of a 0.00476 m hydrochloric acid solution. What is the concentration of [h+] in a solution whose ph = 4.3? What is the poh of a solution that has a. Write the equation for the dissociation of sulfuric acid. Write the equation for the dissociation of hydrochloric acid.
What Is The Poh Of A Solution That Has A.
Write the equation for the dissociation of hydrochloric acid. Write the equation for the dissociation of sulfuric acid. Find the ph of a 0.00476 m hydrochloric acid solution. Calculating ph and poh worksheet w 335 everett community college tutoring center student support services program 1) what is the ph of a 0.0235 m hcl solution?
What Mass Of Naoh Should Be Added To 300.0 Ml Of Water In Order To Prepare A Solution With A Ph = 11.50.
2) what is the poh of a. What is the concentration of [h+] in a solution whose ph = 4.3?

.PNG)



/pHWorksheet-56a12dd95f9b58b7d0bcd1fc.png)